Featured Publications
Molecular Engineering of Conjugated Polymers for Solar Cells: An Updated Report
Shengqiang Xiao, Qianqian Zhang, Wei You;
Adv. Mater. 2017, 1601391; doi:10.1002/adma.201601391
The device efficiency of polymer:fullerene bulk heterojunction solar cells has recently surpassed 11%, as a result of synergistic efforts among chemists, physicists, and engineers. Since polymers are unequivocally the “heart” of this emerging technology, their design and synthesis have consistently played the key role in the device efficiency enhancement. In this article, the first focus is a discussion on molecular engineering (e.g., backbone, side chains, and substituents), then the discussion moves on to polymer engineering (e.g., molecular weight). Examples are primarily selected from the authors contributions; yet other significant discoveries/developments are also included to put the discussion in a broader context. Given that the synthesis, morphology, and device physics are inherently related in explaining the measured device output parameters (Jsc, Voc and FF), we will attempt to apply an integrated and comprehensive approach (synthesis, morphology, and device physics) to elucidate the fundamental, underlying principles that govern the device characteristics, in particular, in the context of disclosing structure-property correlations. Such correlations are crucial to the design and synthesis of next generation materials to further improve the device efficiency.
Valence Band Dependent Charge Transport in Bulk Molecular Electronic Devices Incorporating Highly Conjugated Multi-[(Porphinato)Metal] Oligomers
Robert C. Bruce, Ruobing Wang, Jeff Rawson, Michael J. Therien, Wei You
J. Am. Chem. Soc., 2016, 138 (7), pp 2078–2081; doi:10.1021/jacs.5b10772
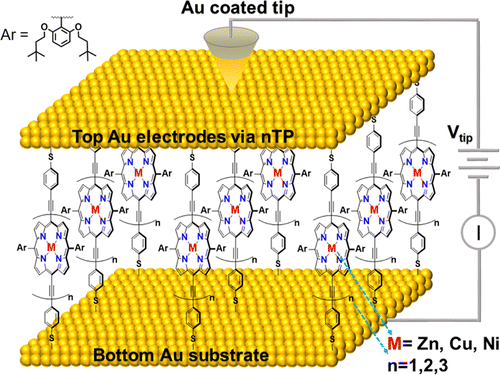
Molecular electronics offers the potential to control device functions through the fundamental electronic properties of individual molecules, but realization of such possibilities is typically frustrated when such specialized molecules are integrated into a larger area device. Here we utilize highly conjugated (porphinato)metal-based oligomers (PMn structures) as molecular wire components of nanotransfer printed (nTP) molecular junctions; electrical characterization of these “bulk” nTP devices highlights device resistances that depend on PMn wire length. Device resistance measurements, determined as a function of PMn molecular length, were utilized to evaluate the magnitude of a phenomenological β corresponding to the resistance decay parameter across the barrier; these data show that the magnitude of this β value is modulated via porphyrin macrocycle central metal atom substitution [β(PZnn; 0.065 Å–1) < β(PCun; 0.132 Å–1) < β(PNin; 0.176 Å–1)]. Cyclic voltammetric data, and ultraviolet photoelectron spectroscopic studies carried out at gold surfaces, demonstrate that these nTP device resistances track with the valence band energy levels of the PMn wire, which were modulated via porphyrin macrocycle central metal atom substitution. This study demonstrates the ability to fabricate “bulk” and scalable electronic devices in which function derives from the electronic properties of discrete single molecules, and underscores how a critical device function—wire resistance—may be straightforwardly engineered by PMn molecular composition.
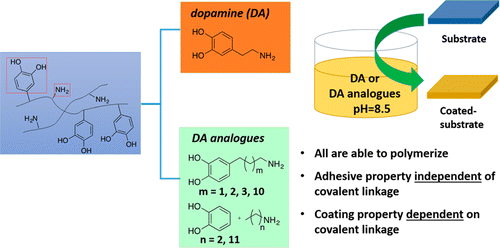
Investigation of Dopamine Analogues: Synthesis, Mechanistic Understanding, and Structure–Property Relationship
Huamin Hu, Jason Christopher Dyke, Brett Allen Bowman, Ching-Chang Ko, Wei You
Langmuir, 2016, 32 (38), pp 9873–9882; doi:10.1021/acs.langmuir.6b02141
Dopamine, perhaps the simplest molecule that covalently links catechol
and amine, together with its derivatives, has shown impressive
adhesive and coating properties with its polymers. However, the scope
of the molecules is rather limited, and the polymerization mechanisms
are still elusive. We designed a general synthetic scheme and
successfully synthesized a series of dopamine analogues with different
alkyl chain lengths between the catechol and amine. Taking these new
dopamine analogues, together with the molecular systems that have
separate catechol and alkyl amine, we show that having both catechol
and amine in the molecular system, whether covalently linked via an
alkyl chain or not, is sufficient to polymerize under a similar reaction
condition to that of dopamine polymerization. However, the
time-dependent UV–vis characterization of the individual polymerization indicates that the polymerization for individual molecular systems likely proceeds via different reaction intermediates, depending on the length of the alkyl chain and whether there is a covalent linkage. Interestingly, whereas the covalent linkage via an alkyl chain is not necessary for showing the adhesive property, it is required to achieve the impressive coating property. Our results offer new insights into the design and synthesis of dopamine analogues for future applications, as well as a further mechanistic understanding of the polymerization of these dopamine analogues.